Research
See our publications on Google Scholar.
The Whitehead Lab has two major thrusts in the area of drug delivery:
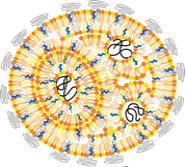
1. RNA delivery and genetic engineering. Nucleic acid drugs, including DNA and many types of RNA, are the medicines of the future, and they will enable personalized therapies that will revolutionize our ability to treat disease. Unfortunately, if you administer these drugs in "naked" form, they are rapidly degraded in the body. Therefore, before these drugs can be FDA approved, we need to develop delivery systems able to package up this sensitive nucleic acid cargo and escort it to the right cells and organ targets in the body. Using our lipid nanoparticle technology, our lab asks questions related to the effect of lipid chemistry on efficacy, toxicity, and immune response. Ultimately, we are interested in using our lipid nanoparticle RNA delivery vehicles to prevent disease through vaccinations and treat diseases that range from Type I diabetes to inflammatory bowel disease.
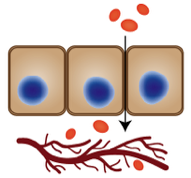
2. Oral delivery of macromolecules. Currently, the only means of administering proteins (e.g. insulin) and other macromolecular drugs is by injection, which is painful and prone to patient non-compliance. Half of our lab focuses on developing the technology needed to deliver protein, peptide, and nucleic acid drugs orally. This is challenging because our gastrointestinal tract is designed to break down these compounds, not absorb them intact. Our lab engineers permeation enhancers, natural products, and polymer-protein sytems to enable safe delivery of protein drugs for therapeutic effect. Learn more about our protein oral delivery work by watching our featured segment on Cheddar.
Although oral delivery is the most patient-friendly route of drug administration, it cannot be utilized for macromolecules because of their susceptibility to enzymatic degradation in the GI tract and low permeability across the intestinal epithelium. We seek to understand the effect of tight junction and epithelial membrane proteins on drug transport and permeation enhancement processes - with a goal of applying this understanding to both the oral delivery of systemic drugs and treatment of inflammatory bowel disease. Click here for a video describing some of our oral delivery work.
Messenger RNA (mRNA) therapeutics have the potential to revolutionize the treatment and prevention of human disease. For example, we can use mRNA for new vaccines, cancer immunotherapy, and gene editing. Our lab develops lipid nanoparticles that can package up mRNA drugs and escort them into target cells. Our lab explores the exquisite control that nanoparticle chemistry has on the delivery process. Our long-term goal is to predict how well particles will work in people based on their chemistry alone.
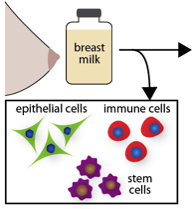
Upon consumption, breast milk cells have been shown to translocate out of the infant's GI tract and functionally integrate into neonatal tissue, where they can persist into the offspring's adulthood. This NIH Director's New Innovator Award project will shed new light on this amazing physiological process and determine how and why breast milk cells exit the GI tract and gain access to the body. We are also engineering breast milk cells to impart therapeutic functionality to the infant. For example, breast milk cells might enable oral vaccination, protein replacement therapy, tolerization to food allergens, and more. Click here for a short video to learn more.
Oral Delivery of Biologic Drugs
Nanoparticle Engineering for mRNA Delivery
Genetic Engineering of Breast Milk Cells for Infant Therapy
Our interests evolve continually, and the projects listed are only a partial representation of the exciting work currently being conducted in our lab!